13 Select patients for therapy based on an FDA-approved companion diagnostic. Trastuzumab Herceptin trastuzumab-pkrb Herzuma trastuzumab-anns Kanjinti trastuzumab-dkst Ogivri and trastuzumab-qyyp Trazimera trastuzumab-dttb Ontruzant.
Https Www Mdbriefcase Com Wp Content Uploads 2019 09 18870 Ad Fr Ogivri Sell Sheet Fr16 07 Pdf
Ogivri typically is used to.

Ogivri j code. The correction and second filing comes as according to the Centers for Medicare and Medicaids CMS most recent ASP report released 5 December 2017 Inflectras average selling price ASP has declined in 2017 while Remicades ASP has increased. HCPCS codes covered if selection criteria are met. 11 12 The treatment of HER2-overexpressing metastatic gastric or.
It is specifically used for cancer that is HER2 receptor positive. Injection trastuzumab-dttb biosimilar Ontruzant 10 mg. Ogivri 420 mg powder for concentrate for solution for infusion One vial contains 420 mg of trastuzumab a humanised IgG1 monoclonal antibody produced by mammalian Chinese hamster ovary cell suspension culture and purified by affinity and ion exchange chromatography including specific viral inactivation and removal procedures.
Biocon Biologics a subsidiary of Biocon has embarked on a journey to cross a revenue milestone of. Ogivri is a cancer medicine used to treat the following conditions. Trastuzumab-dkst is produced by recombinant DNA technology in a mammalian cell Chinese Hamster Ovary culture.
Inj ogivri 10 mg. New code Q5111 adequately describes the product that is the subject of this application and is available for assignment by insurers. Injection trastuzumab-pkrb biosimilar Herzuma 10 mg.
TRASTUZUMAB is a monoclonal antibody. JJ did not respond to a request for comment. Evidence-Based Oncology February 2018 Volume 24 Issue 2.
Biocon and its partner Mylan on December 2 announced the launch of Ogivri - a biosimilar to Herceptin - in USA. OGIVRI trastuzumab is indicated for the treatment of patients with early stage breast cancer with ECOG 0-1 status whose tumours overexpress HER2 following surgery and after chemotherapy following adjuvant chemotherapy consisting of doxorubicin and cyclophosphamide in combination with paclitaxel or docetaxel in combination with adjuvant. Listing of a code in this policy does not imply that the service described by the code is a covered or non- covered health service.
Injection Trastuzumab-dkst biosimilar Ogivri 10 mg. The treatment of HER2-overexpressing breast cancer. Trastuzumab sold under the brand name Herceptin among others is a monoclonal antibody used to treat breast cancer and stomach cancer.
The US launch of Ogivri marks a significant milestone in our biosimilars journey. CMS established new Level II HCPCS code Q5111 Injection Pegfilgrastim-cbqv Biosimilar Udenyca 05 mg effective 112019. Type of Service TOS.
Early breast cancer when the cancer has spread within the breast or to the lymph nodes glands under the arm but not to other parts of the body after surgery chemotherapy medicines to treat cancer and radiotherapy treatment with radiation if applicable. It is an important endorsement of our science development and manufacturing capabilities in the area of monoclonal antibodies said Christiane Hamacher CEO Biocon Biologics. Trastuzumab is given by slow injection into a vein and injection just under the skin.
HER2 human epidermal receptor 2 inhibitor targeted therapy. Type of Service TOS. It may be used by itself or together with other chemotherapy medication.
Ogivri was the first biosimilar of Herceptin to be approved by USFDA two years ago. It is used to treat breast cancer and stomach cancer. This medication is given by slow injection into a.
Ogivri trastuzumab-dkst is a humanized IgG1 kappa monoclonal antibody that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor 2 protein HER2. Ogivri is a HER2neu receptor antagonist indicated for. The following lists of procedure andor diagnosis codes is provided for reference purposes only and may not be all inclusive.
Breast Cancer Gastric Cancer. Treat metastatic HER2-positive breast cancer. Ogivri trastuzumab-dkst is a HER2neu receptor antagonist biosimilar to Herceptin indicated for the treatment of HER2-overexpressing breast cancer and the treatment of HER2-overexpressing stomach cancer.
Last years approval of trastuzumab biosimilar Ogivri will be the first competitor to blockbuster drug Herceptin for. How to use Ogivri 150 Mg Intravenous Solution Antineoplast EGF Receptor Blocker Recomb Monoclonal Antibody. Herceptin Herzuma Kanjinti Margenza Nerlynx Ontruzant Perjeta Phesgo Trazimera and Tykerb are other HER2 inhibitors.
Compare HER2 receptor antagonists. Inj herzuma 10 mg.
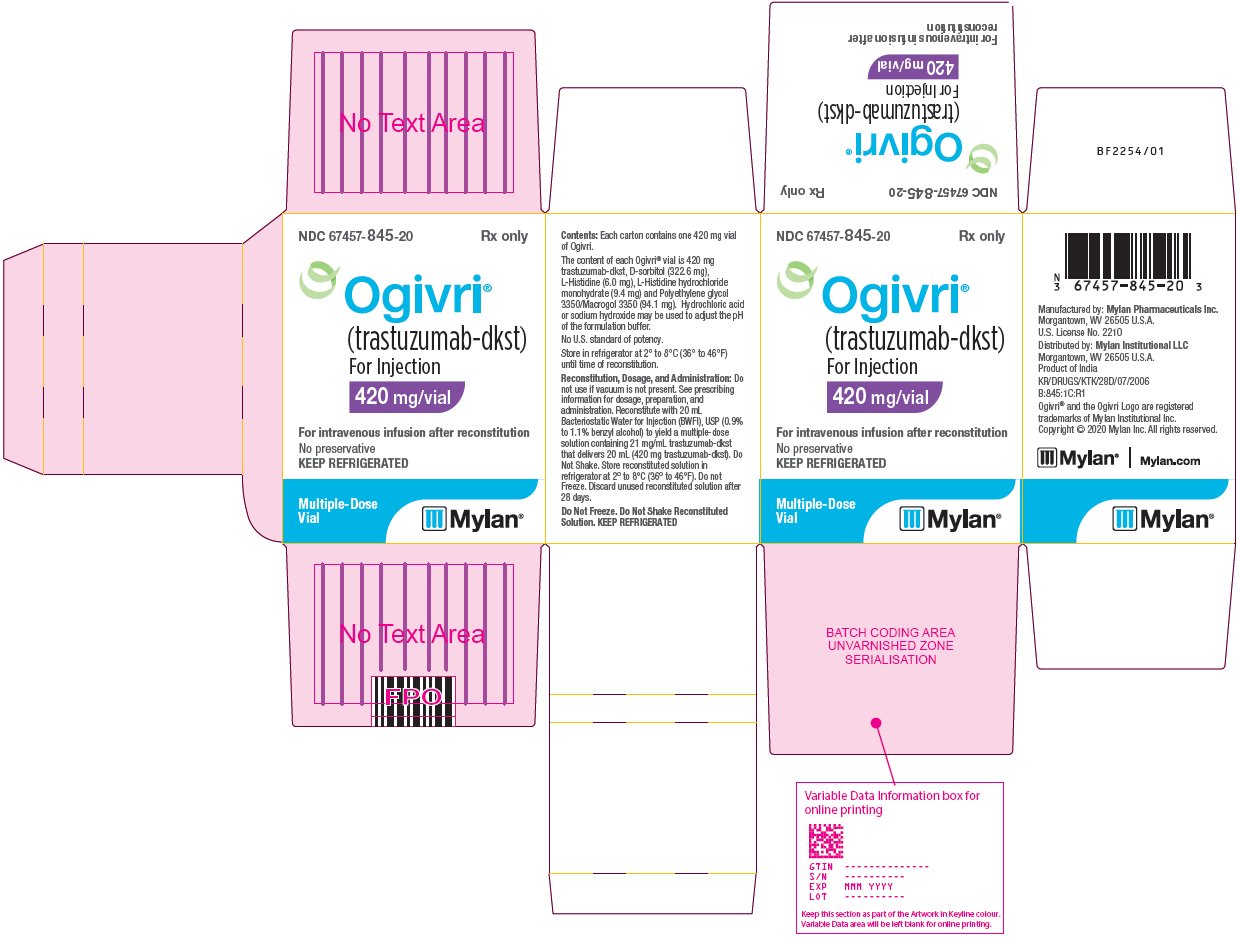 Ndc 67457 847 Ogivri Trastuzumab
Ndc 67457 847 Ogivri Trastuzumab
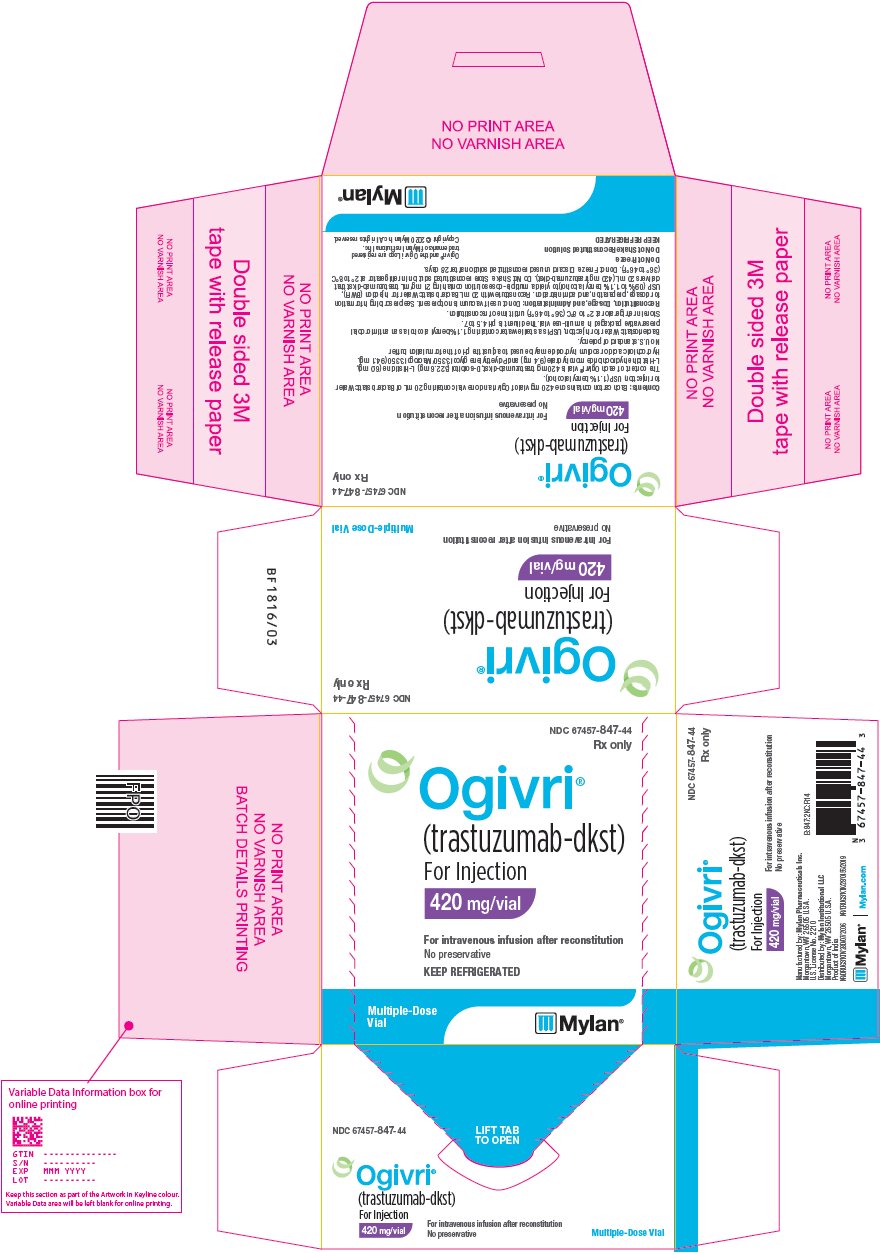 Ndc 67457 991 Ogivri Trastuzumab
Ndc 67457 991 Ogivri Trastuzumab
Http Specialtydrug Magellanprovider Com Media 121338 Ogivri 12 19 Pdf
 Ogivri Injection Fda Prescribing Information Side Effects And Uses
Ogivri Injection Fda Prescribing Information Side Effects And Uses
 Ogivri Injection Fda Prescribing Information Side Effects And Uses
Ogivri Injection Fda Prescribing Information Side Effects And Uses
Https Www Mdbriefcase Com Wp Content Uploads 2019 09 18870 Ad Fr Ogivri Sell Sheet Fr16 07 Pdf
Https Www Mdbriefcase Com Wp Content Uploads 2019 09 18870 Ad Fr Ogivri Sell Sheet Fr16 07 Pdf
 Health Canada Approves Mylan And Biocon S Ogivri The First Trastuzumab Biosimilar For The Treatment Of Her2 Positive Breast And Gastric Cancers
Health Canada Approves Mylan And Biocon S Ogivri The First Trastuzumab Biosimilar For The Treatment Of Her2 Positive Breast And Gastric Cancers
Https Www Mdbriefcase Com Wp Content Uploads 2019 09 18870 Ad Ogivri Sell Sheet English 16 7 2019 Pdf
 Samsung Bioepis And Merck Launch Trastuzumab Biosimilar In The United States Onco Zine
Samsung Bioepis And Merck Launch Trastuzumab Biosimilar In The United States Onco Zine
Https Www Mdbriefcase Com Wp Content Uploads 2019 09 18870 Ad Ogivri Sell Sheet English 16 7 2019 Pdf



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.